Information
Table of Contents
Frequently Asked Questions
General Questions
- Q: What is the purpose of the USDA BioPreferred® Program?
A: The Program's purpose is to spur economic development, create new jobs, and provide new markets for farm commodities. By harnessing the powers of certification and the marketplace, the Program helps purchasers and users identify products with biobased content and assures them of its accuracy. - Q: What is a "biobased" product?
A: A biobased product is a commercial or industrial product (other than food or feed) that is composed, in whole or in significant part, of biological products, including renewable domestic agricultural materials (including plant, animal, and aquatic materials), forestry materials, intermediate materials, or feedstocks. Biobased products exclude motor vehicle fuels, heating oil, or electricity produced from biomass. - Q: How do I determine the biobased content of a product?
A: Biobased content may be estimated using the ratio of "new" organic carbon (such as from agricultural materials) to total organic carbon ("new" + "old" or petroleum-based carbon). Water and inorganic carbon (such as carbonate) are excluded. This document provides more information about biobased content and how it is determined. The Program uses ASTM D6866 to quantify the biobased content of a product during the certification process.
For Companies
Getting Started
- Q: In which of the USDA BioPreferred Program's initiatives may I participate?
A: The BioPreferred Program has two major initiatives: mandatory federal purchasing and voluntary labeling. A product that fits into one of the 139 categories identified for mandatory federal purchasing has two options for participating: (1) As a qualified product under the mandatory federal purchasing initiative, or (2) As a qualified product under the mandatory federal purchasing initiative and as a certified product approved to display the USDA Certified Biobased Product label under the voluntary labeling initiative. If a product does not fall into one of the 139 categories identified for mandatory federal purchasing, then it may participate as a certified product that has been approved to display the USDA Certified Biobased Product label.
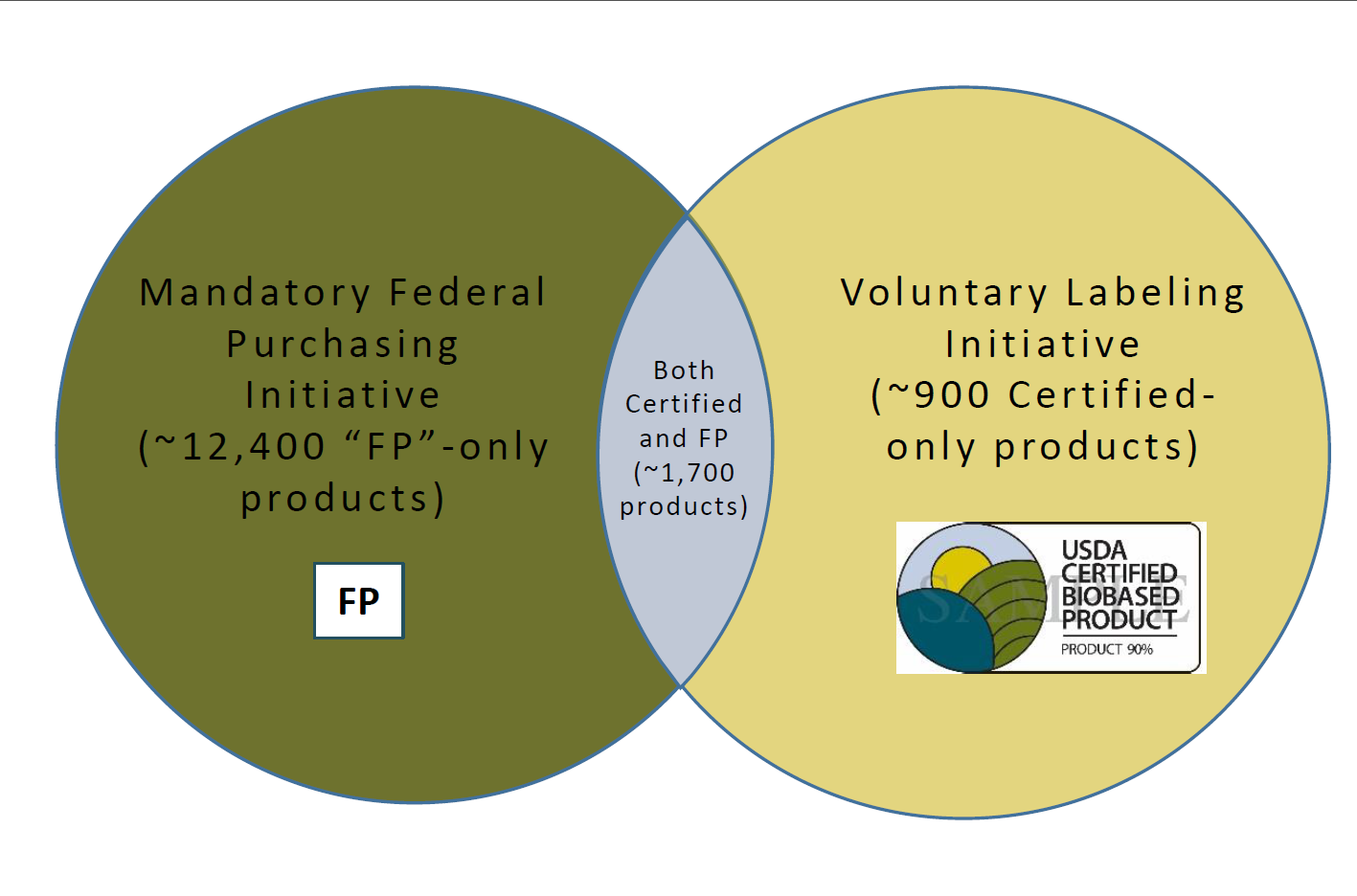
- Q: How do the two initiatives differ?
A: A manufacturer with a product qualified for preferred federal purchasing is encouraged to market it to the federal Government. This typically requires partnering with an approved federal contractor that provides services to the federal Government and can use biobased products in delivering those services (such as janitorial services, or construction and building maintenance contracts). The USDA Certified Biobased Product label adds credibility to product claims, helps counter greenwashing practices and is highly sought after by consumers during the buying process. Of course, biobased products that qualify for federal purchasing preference may also become certified, further emphasizing a company's commitment to sustainability. - Q: What are the steps to have a product certified?
A: For a product to become a USDA Certified Biobased Product, a specific, federally regulated list of steps must be followed. Skipping or completing any steps out of order will prevent the product from becoming certified. To get started, please use the Company Tools Tutorial. - Q: What are the eligibility requirements
for the USDA Certified Biobased
Product label?
A: Biobased products that fit within one of the 139 product categories identified by the USDA for mandatory federal purchasing must meet or exceed the minimum biobased content requirement for that product category. Products that do not fall under one of these product categories must be at least 25% biobased. Biobased products must also meet the Programs Innovative Criteria to participate. - Q: How much does it cost to participate in the Program?
A: At this time there is no fee for manufacturers or distributors to participate in the Program, certification, labeling, or application process. Companies are responsible for the cost of biobased content testing required during the label application process and periodic re-testing during audits. Product testing is completed using ASTM D6866, which costs approximately $400 USD per sample, plus shipping costs. - Q: When applying for the USDA Certified Biobased Product label,
is it required that the (agricultural) raw materials be grown in the U.S.?
A: No. Companies from more than 50 countries currently participate in the Program. As long as the country in question is allowed to trade with the U.S. (see this page for more information), companies from that country may participate in the Program. - Q: Does USDA provide grant assistance to help defray the costs of testing?
A: The USDA BioPreferred Program does not provide such assistance, but some states may have, or may be in the process of developing, assistance programs for supporting companies who manufacture biobased products. The USDA Rural Development Program may also have grants available to biobased product manufacturers. - Q: If my product is qualified through the mandatory federal purchasing initiative,
do I still have to apply for the certification label, or is it automatic?
A: Even if your product already participates in the mandatory federal purchasing initiative, you must still apply for certification to use the USDA Certified Biobased Product label. - Q: If there is no product category already established for my product, what do I do?
A: You can still submit an application for certification in the "Other" product category, but your product will not be eligible for mandatory federal purchasing. Products for which there is no minimum biobased content already established must be at least 25% biobased. You may also fill out this form if you wish to suggest a new category. Suggestions will be considered in future product category designations, and if selected, may be designated for mandatory federal purchasing in a few years. -
Q: I am having trouble accessing my account.
A: Please follow the instructions in the Troubleshooting Account Access Tutorial. -
Q: I need to access an existing company account.
A: Please follow the instructions in the Company Account Access Tutorial in order to create your own user account and access an existing company account.
The Certification Process
- Q: How long does the approval process take once I apply for the USDA Certified Biobased Product label?
A: The entire certification process, from application through testing, generally takes about 90 days, if companies provide thorough, prompt responses to Program inquiries. When companies do not reply to inquiries, time to certification becomes indefinitely longer. Given the wide range of factors that influence time to certification, be aware that we cannot guarantee certification by a certain date. - Q: Can my application be expedited?
A: In the interest of fairness to all of our participants, we do not expedite applications. Applications are processed in the order they are received, usually within 60 days. However, there are some things you can do to make the process as efficient as possible. Following the instructions in the Company Tools Tutorial for submitting your application, and providing quick, thorough answers to any follow-up questions our team has about your application makes the process go faster. - Q: How are applications for the USDA Certified Biobased Product label evaluated?
A: USDA and its contractor, Savan Group, will review each application. They will contact the company with any additional questions before rejecting or pre-qualifying the application. Applications are evaluated on accuracy and thoroughness of content, overall fit with the Program, and eligibility of the product in question. - Q: If I've already had my products tested with one of the approved laboratories,
can I use those test results to obtain certification?
A: No. Only products tested using the order outlined here, as specified by the Program guidelines,are eligible for certification. If a product was tested outside the Program, it will need to be re-tested at the proper time during the certification process to receive certification. - Q: What if my company sells two or more products made of the same or
similar formulation(s)?
A: If products in the same company account have similar formulations (their biobased contents are within +/-3% of one another), they may be placed in a product family together. The biobased content of only one product per family must be tested. Variations of the same product (different sizes, colors, or scents) are not considered to be separate products and should not be added to a product family together. These should be listed as variations of a single product when prompted in the application. Test-exemptions are used when the same formulation is sold by two separate companies. See question #6 for information on test-exemptions. - Q: What if my USDA Certified Biobased Product is sold under another
company's brand (private labeling)?
A: If a product is identical in formulation to an already-certified product sold by another company, it may be exempt from testing. The company marketing and selling the product should create an account and submit a test-exempt application to the Program. They may receive certification for their product through this method if the manufacturer chooses to share their certification. Testing with ASTM D6866 is not required for this process. - Q: Where can I get a list of approved testing laboratories?
A: A list of approved laboratories is available on the BioPreferred Program website here. - Q: Where can I find additional information
about the sampling, test specimens, and testing required for the
Program's voluntary labeling initiative?
A: You can contact the USDA BioPreferred Program team at help@usdabiopreferred.net or directly contact the lab where you plan on having your sample tested; the labs' contact information can be found here. - Q: Is third party verification of biobased content required for a product
to be displayed in the BioPreferred Program's catalog?
A: USDA does not require manufacturers to provide third party verification of biobased content in order to be listed in the catalog through the mandatory federal purchasing initiative. The manufacturer must self-certify that they meet the minimum biobased content set for their product's category in order to be included in the catalog. However, a federal agency considering the purchase of your product may require third party verification. Products listed in the catalog through the voluntary labeling initiative have received third party verification of their biobased contents as part of the certification process.
Once your product is certified
- Q: What is the auditing process for products or packages that obtain the USDA Certified Biobased Product label?
A: USDA will routinely audit USDA Certified Biobased Products and Packaging to ensure that these products and packaging contain the biobased content that is displayed on the label. -
Q: How can I use the label on my website/ advertising/ marketing?
A: The use of BioPreferred Program terminology and the label are closely regulated by the USDA. Please refer to the Brand Guidelines before distributing information about the Program or your company's involvement in the Program. Any violations found by Program staff should be addressed promptly, or the company in question may have its certification revoked. - Q: What should I do if I change the name of my product or company?
A: Any company or product name changes must be documented in the online system. Please follow the Program's product and company name change procedures. - Q: What should I do if I change the formulation of my product?
A: If the formulation of a certified product changes please contact the BioPreferred Program's support staff and provide details about the type and magnitude of the change. Product retesting may be required. - Q: How do manufacturers/vendors make federal agencies aware of the
qualified products they have to offer?
A: The first step is to add a product in the Program's database. If approved as a product qualified for mandatory federal purchasing, it will appear in the USDA BioPreferred Program Catalog. There are also some resources to help companies learn how to sell biobased products to the federal government on the Training and Education Resources page.
For Consumers
- Q: Are biobased products plant-based?
A: The BioPreferred Program is a single-attribute program that focuses exclusively on biobased content. Biobased content is different from plant-based content because biobased content can be sourced from animal or marine sources as well as from plants. Although many biobased products do contain ingredients derived from plants, participation in the BioPreferred Program cannot be used to substantiate the claim that a product is plant-based. For more information on environmental marketing claims please refer to the federal Trade Commission's Green Guides. - Q: Are products that display the USDA Certified Biobased Product label independently tested for biobased content?
A: Yes, products are tested for biobased content by an ASTM-certified third party lab to verify the biobased content in the product using ASTM D6866. The standard is cited in federal law (7 CFR part 3201.7) and is internationally recognized. - Q: Are biobased products "safer" or "better" for the environment than non-biobased
products?
A: The USDA Certified Biobased Product label is not a guarantee or warranty of safety or an expression of environmental preference or impact. The increased use of biobased products is expected to help reduce petroleum consumption by increasing the use of renewable resources, reducing the amount of additional carbon released into the atmosphere. This may help to better manage the carbon cycle, and in turn, reduce resultant adverse environmental and health impacts. For more information on environmental marketing claims please refer to the Federal Trade Commission's Green Guides. - Q: Does a higher percentage of biobased content mean a product is "better"?
A: Biobased content percentages indicate the percentage of renewable, "new" organic carbon in a product. More renewable organic carbon does not necessarily indicate superior performance, safety, or environmental impact. - Q: Do biobased products earn points related to LEED certification?
A: Biobased products may help to achieve Materials and Resources Credits 6 (MR 6) as well as additional credit categories. Using products that are biobased, in addition to meeting other LEED criteria, may earn 1 LEED point. See the LEED website for more information.
For Federal Contractors and Contracting Officers
- Q: Which federal agencies are required to participate in this program?
A: Federal Law requires all federal agencies including the Department of Defense and federal agency contractors to give preference to biobased products within the 139 categories that have been designated for mandatory federal purchasing. - Q: What types of contracts require biobased products?
A:The 139 categories that have been designated for preferred federal purchasing cover a wide range of products, including products used in construction, renovation, groundskeeping, roads and parking lots, janitorial services, facilities operations and maintenance, fleet management, food service, cafeterias, industrial settings, and offices. You can find sample language to paste into your solicitation here. - Q:Where can my contract mention biobased products?
A: Your solicitation can contain the full text of Clause 52.223-2 Affirmative Procurement of Biobased Products Under Service and Construction Contracts. Sections C, F, L, and M can all include biobased products. - Q:Where can I find biobased products to purchase?
A: All USDA Certified Biobased Products and qualified biobased products are listed in the Program's online catalog. Products cannot be directly purchased from the catalog, but participants have often provided links to sites where their products are available for sale. There are also a number of other methods for locating biobased products, including GSA Advantage!, the GSA/GPC Sustainable Facilities (SF) Tool, the Ability One catalog, and the Fed Mall. - Q:How can I determine if a biobased product meets my
specifications?
A: : Use the USDA BioPreferred Program Catalog search bar to find the standard you are looking for. For example, entering, "Safer Choice" into the search bar will return all biobased products in the catalog that also meet EPA's Safer Choice criteria (if the manufacturer chose to share that information). - Q: How do I report biobased purchases, and who should I contact if I have
questions about reporting?
A: Biobased product purchases are reported through the System for Award Management (SAM). You can find more information about reporting on their website and can contact their help desk with any questions. - Q: When must I report my biobased product spending to my contracting
officer and the SAM?
A: We recommend reporting biobased purchases throughout the year as the purchases are made. Spending for the previous fiscal year (October 1st through September 30th) is required to be reported by October 31st of each year. - Q: My contractor is unable to report their biobased
product purchasing in the SAM. What is the problem?
A: The 8L (Environmental Considerations) data field in the Federal Procurement Data System – Next Generation (FPDS-NG) must be correctly coded for a contractor to report biobased products purchasing. One of the following codes must be entered into FPDS-NG field 8L when the contract is awarded: E, H, J, K, or L. - Q: Who should I contact if I still have questions
about biobased product purchasing or reporting requirements? What if my contract requires
biobased product purchase reporting but I have not purchased any biobased products?
A: Your contracting officer or supervisor. The Training and Education Resources page of our website may also have helpful information. - Q: Should motor-vehicle fuels using an ethanol
blend be reported?
A: No, motor-vehicle fuel is excluded from the BioPreferred Program. However, neat biodiesel, also referred to as B100, can be reported when used as an additive. When neat biodiesel is used as a fuel or blended biodiesel fuel, it is excluded. - Q: Where can I learn more about incorporating
biobased purchasing requirements in service and construction contracts? Will I get CLPs?
A: The Training and Education Resources page has courses aimed specifically to assist Federal audiences understand biobased products and how to boost sustainable acquisition efforts. Some courses have recommended CLPs. - Q: When will proposed rules be available to designate additional product
categories for a Federal Procurement Preference?
A: The designation of additional categories and rules for mandatory federal purchasing is mandated by the federal government, and will be published in the CFR, future Farm Bills, Executive Orders, or other legislation. New categories will be published after a sufficient amount of information has been collected on products within each category. - Q:Which sections of the Federal Acquisition
Regulation (FAR) reference or apply to biobased product purchasing requirements?
A: FAR sections 2 – Definitions; 7 – Acquisition Planning; 11 – Describing Agency Needs; 13 – Simplified Acquisition Procedures; 23 – Environment, Energy and Water Efficiency, Renewable Energy Technologies, Occupational Safety, and Drug-free Workplace; 42 – Contract Administration; and 52 – Solicitation Provisions and Contract Clauses.
